

Cancer Risk Test [Proteo®]
Cancer statistics
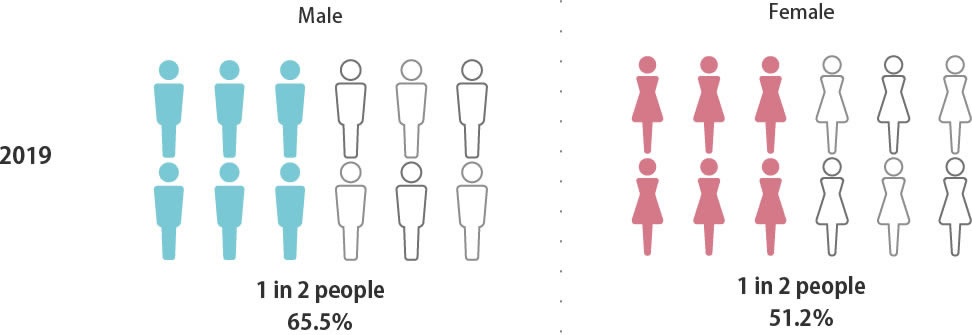
Probability of a Japanese person
being diagnosed with
cancer in his or her lifetime
being diagnosed with
cancer in his or her lifetime
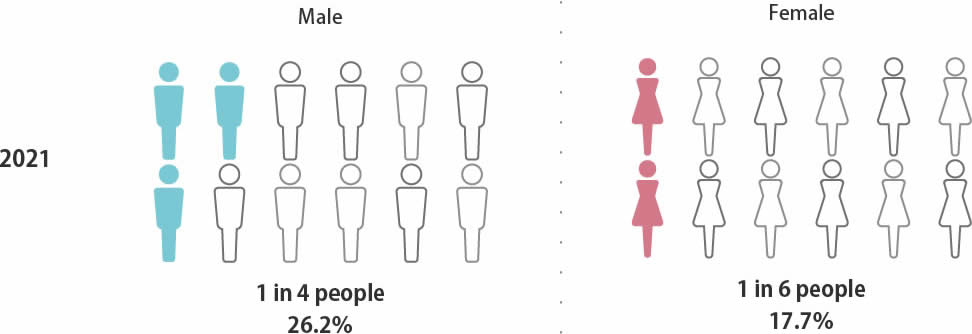
Probability of death
from cancer in Japan
from cancer in Japan
Ranking of cancer incidence (2019)
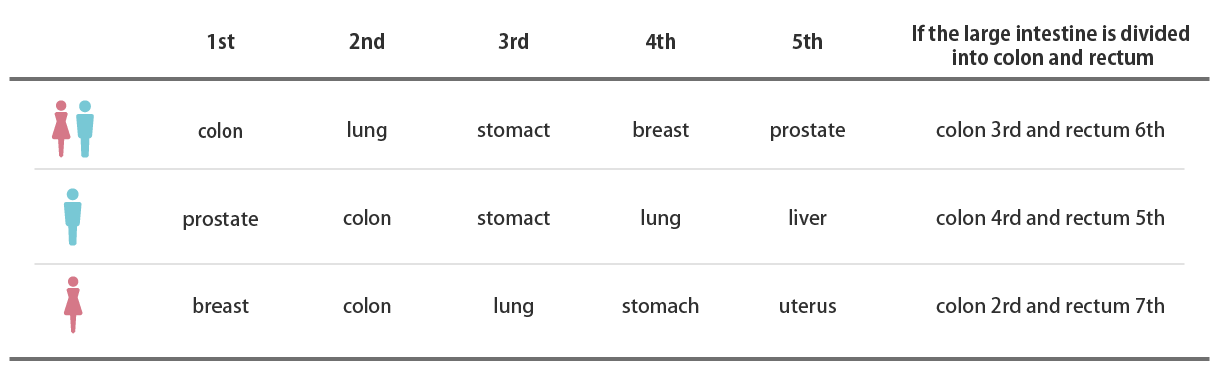
Source: Cancer Information Service Home Page
Summary of the latest cancer statistics Ranking of cancer incidence (2019)
Summary of the latest cancer statistics Ranking of cancer incidence (2019)
Ranking of cancer deaths (2021)
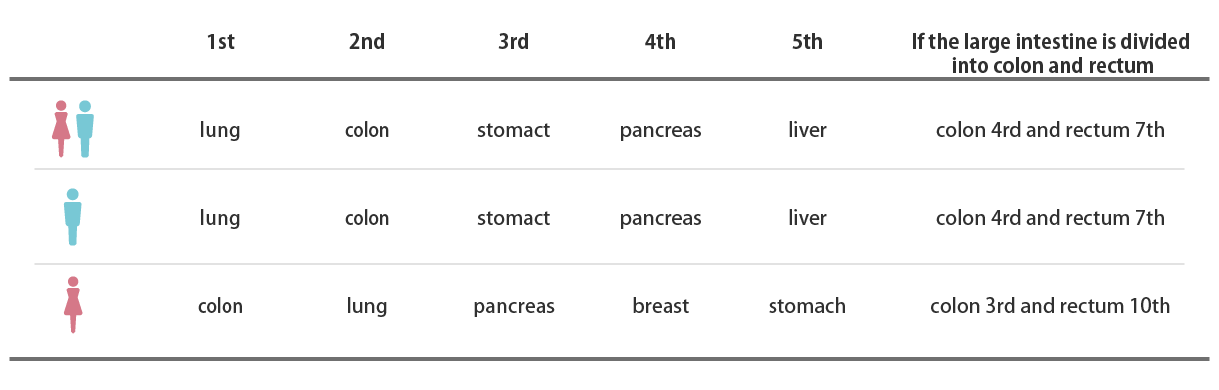
Source: Cancer Information Service Home Page
Summary of latest cancer statistics Ranking of cancer deaths (2021)
Summary of latest cancer statistics Ranking of cancer deaths (2021)
Changes in the number of cancer deaths
Since 1981, cancer has become the number one cause of death, and currently one in three people die from cancer.
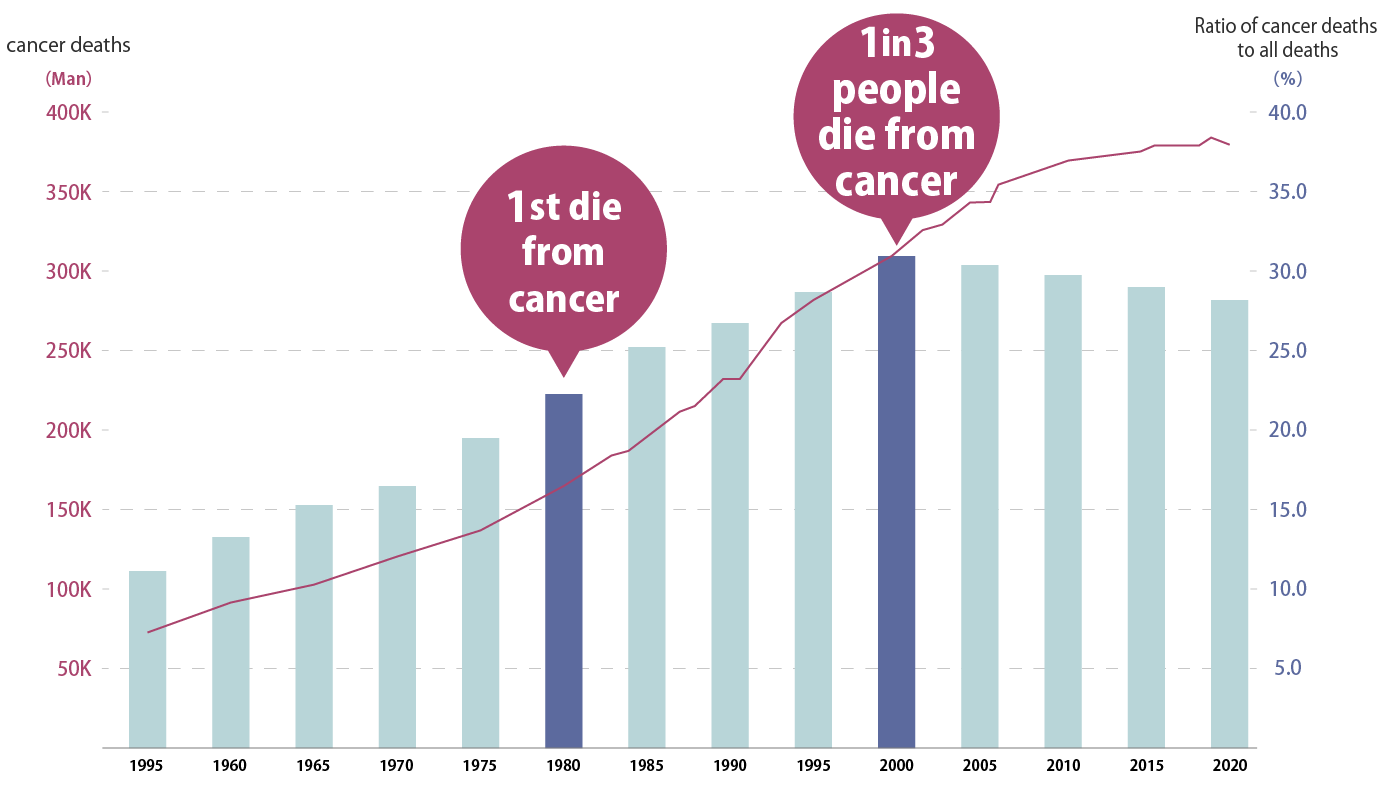
Source: Cancer control promotion corporate action Home Page
Cancer trends: Changes in the number of cancer deaths
Cancer trends: Changes in the number of cancer deaths
Cancer screening rate in Japan compared to other countries
The screening rate in Japan is one of the lowest in the world.
It is about 40 points lower than the United States.
It is about 40 points lower than the United States.
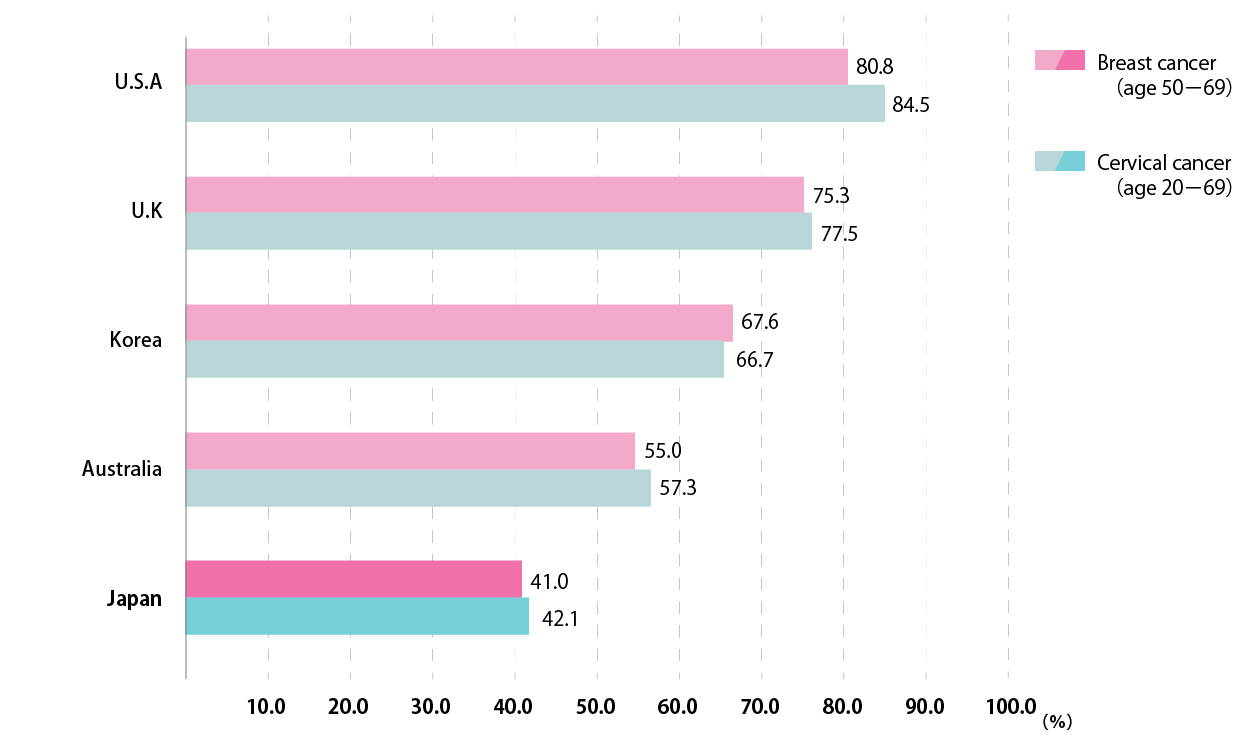
Source: Osaka International Cancer Center Cancer Control Center Home Page
Early detection of cancer through cancer screening Fig. 1 Cancer screening rate in Japan compared with other countries
Early detection of cancer through cancer screening Fig. 1 Cancer screening rate in Japan compared with other countries
Reasons for not undergoing cancer screening
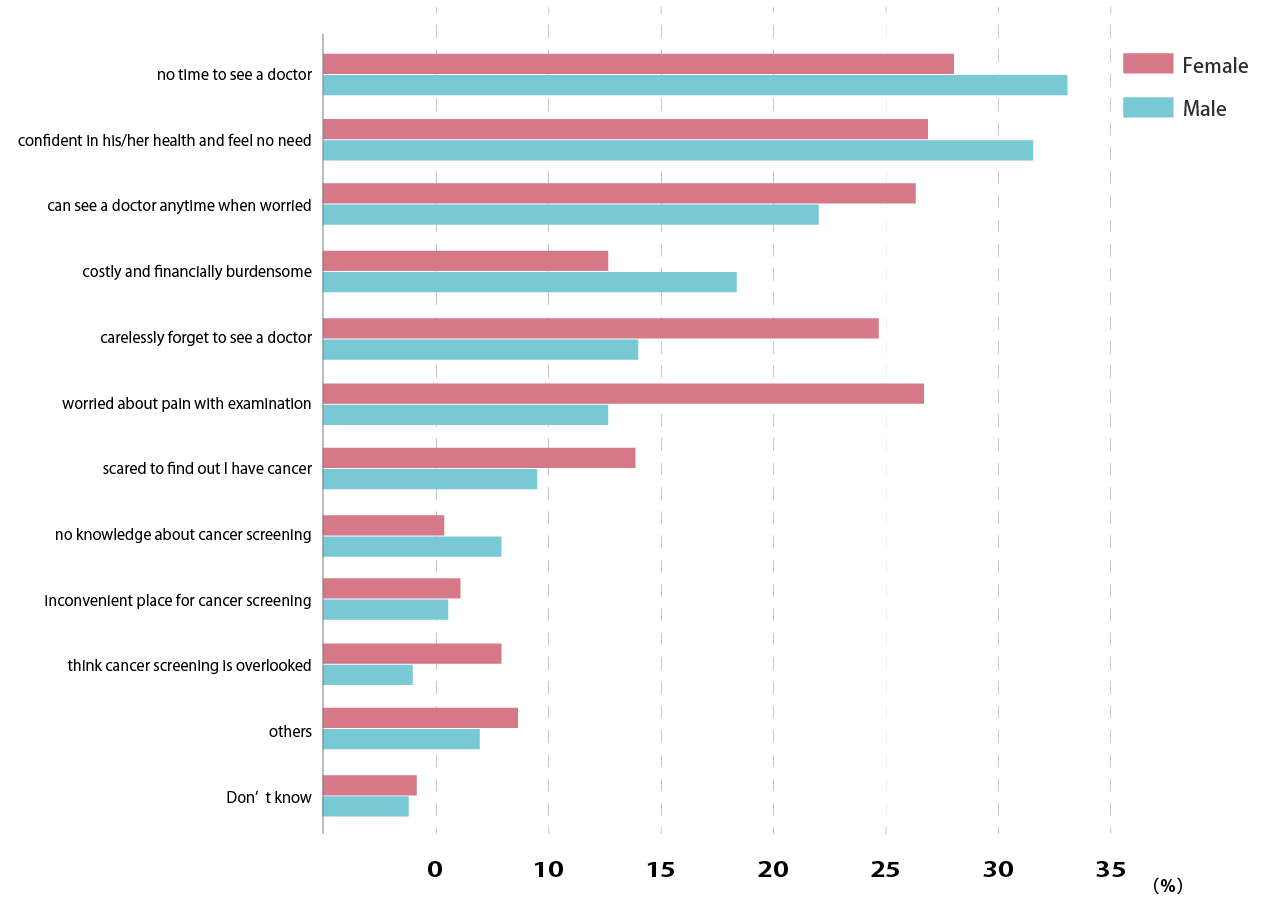
Source: Osaka International Cancer Center Cancer Control Center Home Page
Early detection of cancer through cancer screening Fig. 2 Reasons for not undergoing cancer screening
Early detection of cancer through cancer screening Fig. 2 Reasons for not undergoing cancer screening
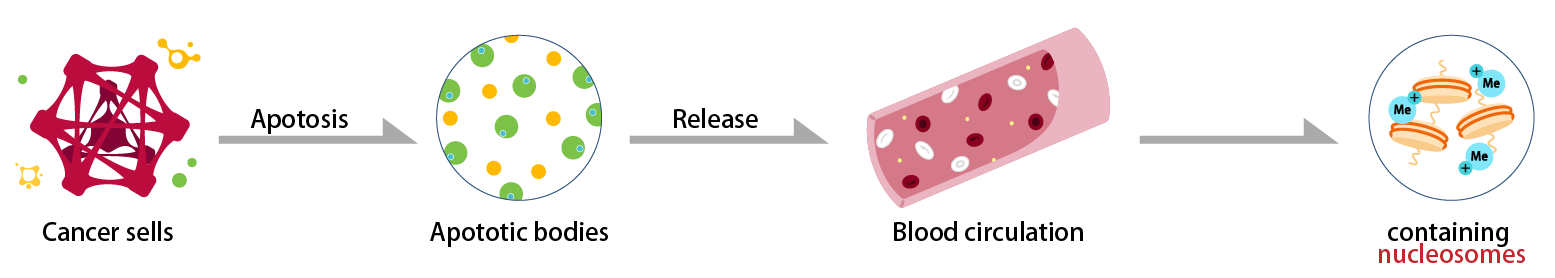
Cancer cells
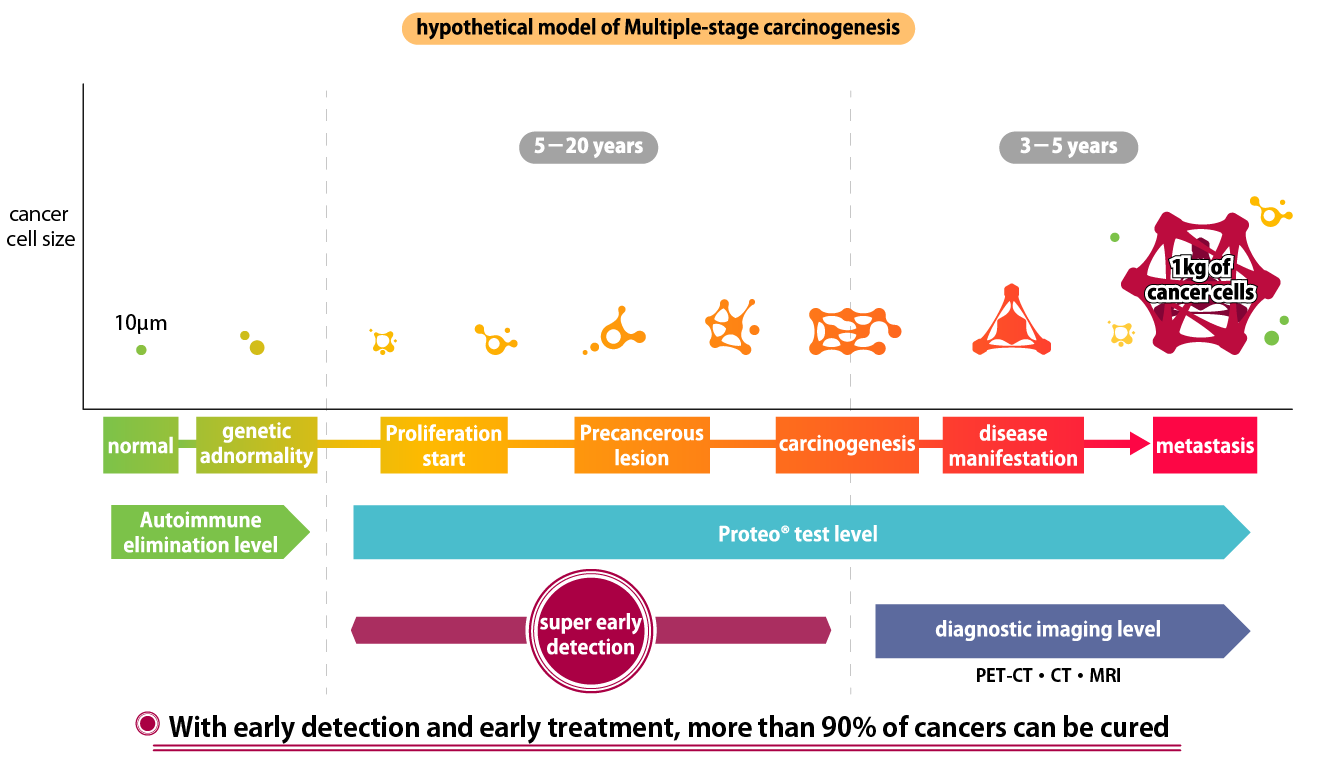
Cancerous cells are eliminated by apoptosis (cell death) caused by autoimmunity, but cancer cells that exceed the level of autoimmunity begin to proliferate and grow larger.
Apoptotic cancer cells leak fragmented nucleosomes into the blood.
Apoptotic cancer cells leak fragmented nucleosomes into the blood.
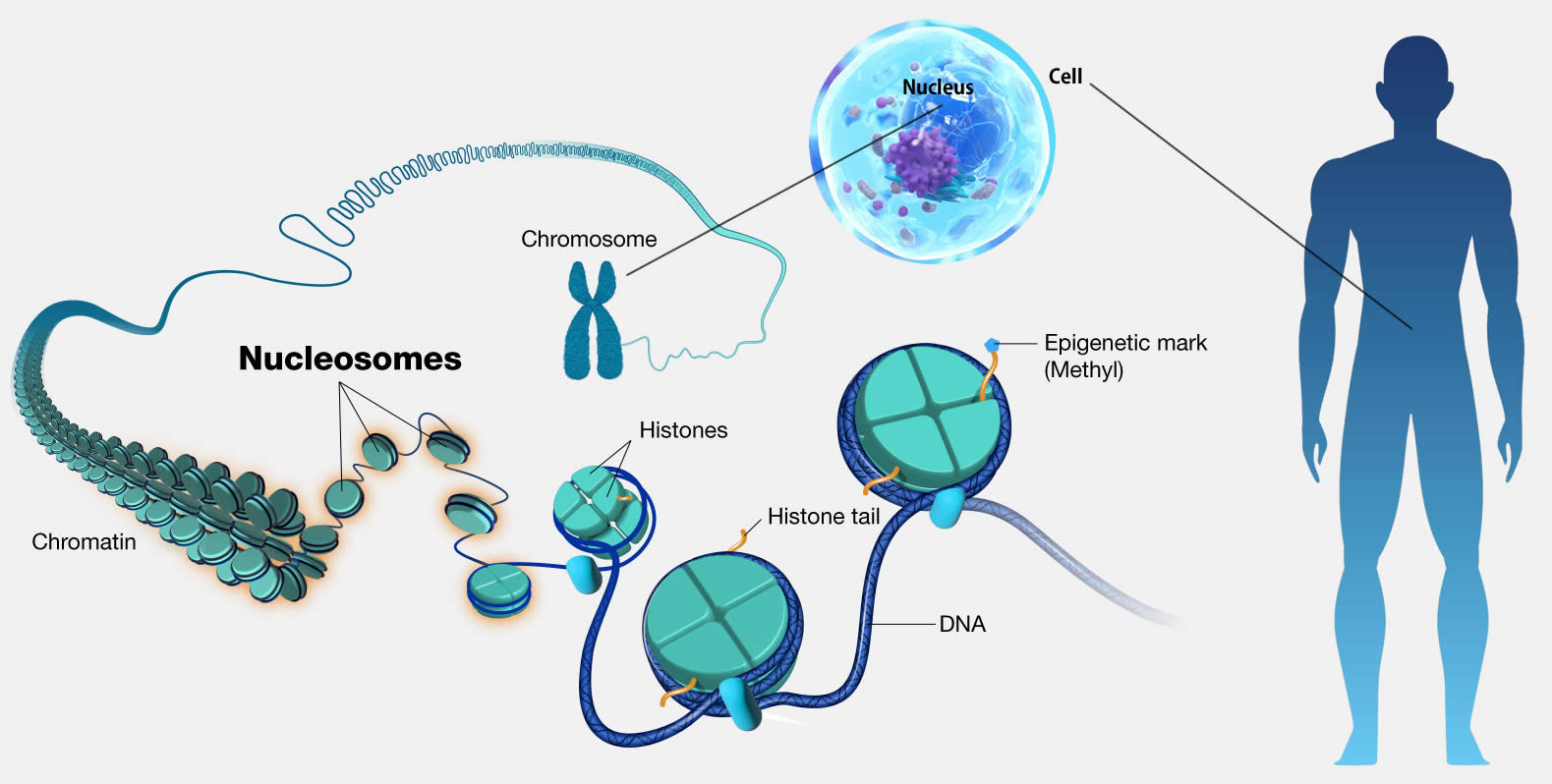
Nucleosome
Nucleosomes are the basic structural units of chromosomes, and are complexes in which DNA is wrapped 1.75 times around proteins called histones.
Through epigenetics (acquired chemical modification), chemical modification such as DNA methylation, histone methylation, acetylation, etc. has occurred.
Through epigenetics (acquired chemical modification), chemical modification such as DNA methylation, histone methylation, acetylation, etc. has occurred.
Proteo®Biochip

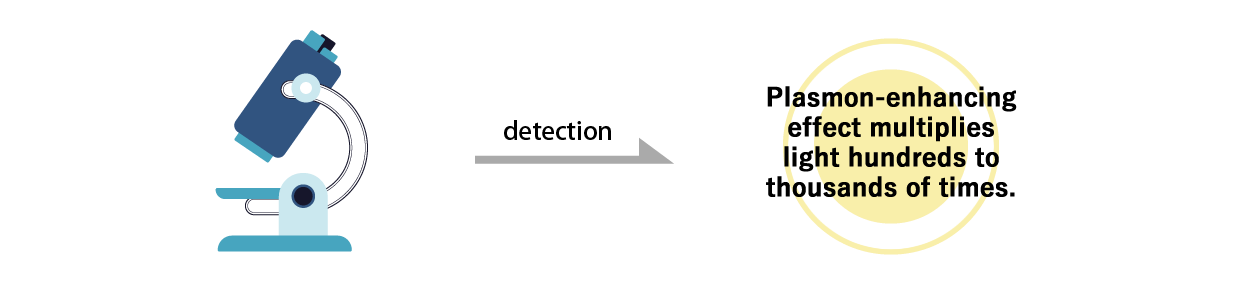
As "Proteo® Biochip" has the feature that the silver peroxide meso-crystals enhance weak autofluorescence, the autofluorescence of nucleosomes on the chip can be measured without labeling (as it is).
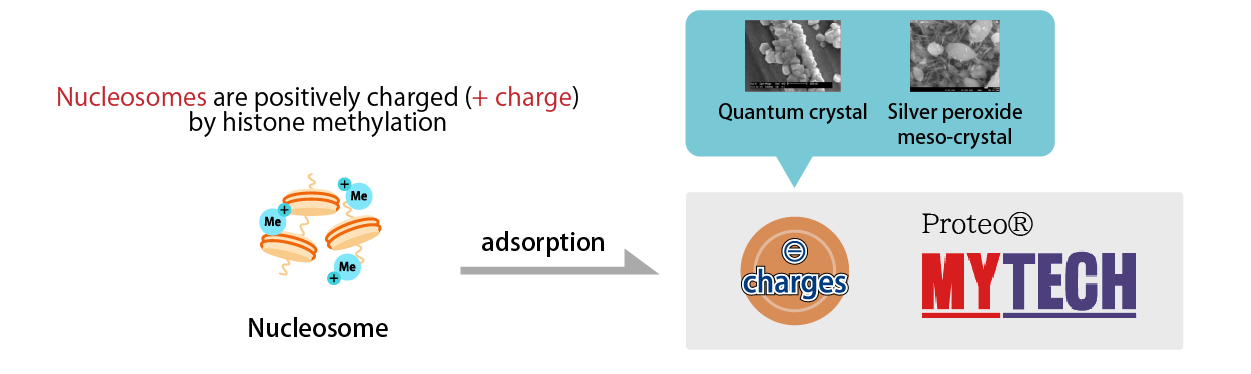
In cancer cells, histones are frequently methylated, and the methylation makes the nucleosomes positively charged (plus).
The "Proteo® Biochip" is made of a novel material called silver peroxide meso-crystal, which is a charge-converted quantum crystal, and the surface of the silver peroxide meso-crystal (plasmonic material) is negatively charged.
Therefore, it can specifically attract positively charged nucleosomes like a magnet.
The "Proteo® Biochip" is made of a novel material called silver peroxide meso-crystal, which is a charge-converted quantum crystal, and the surface of the silver peroxide meso-crystal (plasmonic material) is negatively charged.
Therefore, it can specifically attract positively charged nucleosomes like a magnet.
Specify wavelength of malignant tumor (cancer) and benign tumor

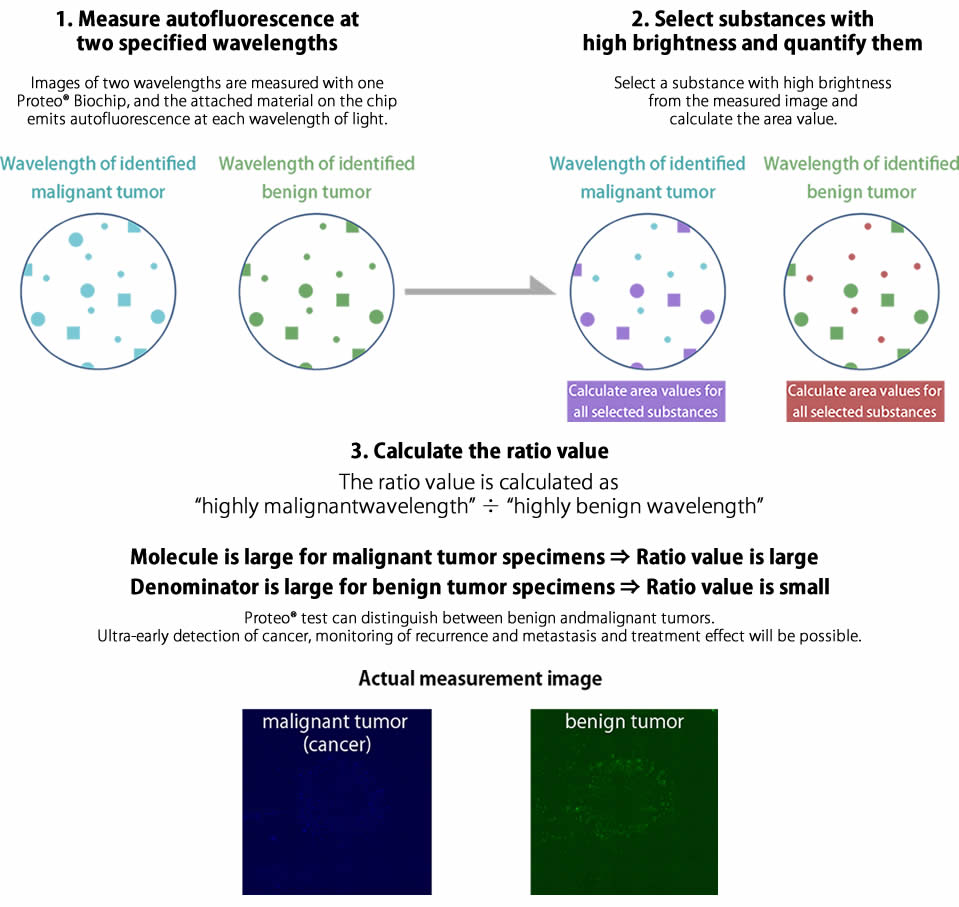
Proteo®Biochip Blind Clinical Trials
From July 2017 to February 2022, Proteo® conducted a 5-year follow-up blind study comparing membership physical examinations.
Because membership-based physical examinations can be followed regularly, a 5-year follow-up survey was conducted after the blind study, and the results of the clinical study were extremely accurate when compared with the definitive diagnosis. (Total 95 specimens)
Because membership-based physical examinations can be followed regularly, a 5-year follow-up survey was conducted after the blind study, and the results of the clinical study were extremely accurate when compared with the definitive diagnosis. (Total 95 specimens)
- MYTECH
(Proteo®Trials)
(Proteo®Trials)
Percentage of correct answers
87.4% (83/95)
Cancer detection rate
2.1% (2/95)
Positivity rate
About 8% (8/95)
- Health Screening Facilities in Tokyo
(Membership physical examinations)
(Membership physical examinations)
Percentage of correct answers
84.2% (80/95)
Cancer Detection Rate
4.2% (4/95)
Positivity rate
About 16% (15/95)
Cancer screening (national average) 2028
Average cancer detection rate 0.142%
Average cancer detection rate 0.142%
| Cancer Screening Type | Cancer Detection Rate | Positive Response Median | Subjects (FY 2008) | Method of Examination |
|---|---|---|---|---|
| Gastric Cancer Screening | 0.122% | 1.70% | Total 50-74 Male/Female | Gastric X-ray |
| Colorectal Cancer Screening | 0.197% | 3.20% | Total 40-74 Male/Female | Fecal Occult Blood Test |
| Lung Cancer Screening | 0.042% | 2.40% | Total 40-74 Male/Female | Chest X-ray (including in combination with sputum cytology) |
| Breast Cancer Screening | 0.320% | 5.20% | Total 40-74 Male/Female | Mammography |
| Cervical Cancer Screening | 0.028% | 1.30% | Total 20-74 Male/Female | Cytodiagnosis |
Cancer Screening (National Average) Report on Community Health and Health Promotion Programs, Fiscal Year 2028
Source: "Cancer Information Service," Cancer Screening Process Indicators by Prefecture
Source: "Cancer Information Service," Cancer Screening Process Indicators by Prefecture
*What is the percentage of correct answers?
The rate at which the test can correctly discriminate between healthy individuals and cancer, and the high percentage of correct responses is the accuracy of the test.
*What is the cancer detection rate?
This is the percentage of those who underwent cancer screening who were found to have cancer.
It is a measure of whether cancer is detected at an appropriate frequency through screening, with higher values being more desirable.
If the cancer detection rate is low, it is possible that many cancers are missed (false negative: a person who is originally positive is mistakenly tested negative).
*What is positive rate?
It is the percentage of positive identifications in that test.
If the positive rates of the tests are aligned at 16%, the detection rates of the Proteo® test and the membership physical exam are equivalent.
The rate at which the test can correctly discriminate between healthy individuals and cancer, and the high percentage of correct responses is the accuracy of the test.
*What is the cancer detection rate?
This is the percentage of those who underwent cancer screening who were found to have cancer.
It is a measure of whether cancer is detected at an appropriate frequency through screening, with higher values being more desirable.
If the cancer detection rate is low, it is possible that many cancers are missed (false negative: a person who is originally positive is mistakenly tested negative).
*What is positive rate?
It is the percentage of positive identifications in that test.
If the positive rates of the tests are aligned at 16%, the detection rates of the Proteo® test and the membership physical exam are equivalent.
Blind clinical trial
Implementation
period
period
February in 2022
Test method
Prospective blind clinical trial
Implementation
medical facility
medical facility
Medical institutions in Tokyo
Number of cases
10 cases
Test method
MYTECH Liquid Biopsy test.
Specimens (serum) are transported refrigerated and tested in Kobe.
Specimens (serum) are transported refrigerated and tested in Kobe.
* Prospective test
A test that collects data from the present to the future
* Retrospective test
A test that collects historical data
* Liquid Biopsy test
Tests using bodily fluids such as blood, urine, and saliva
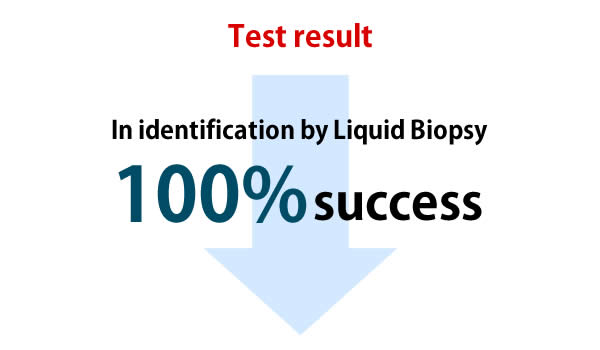
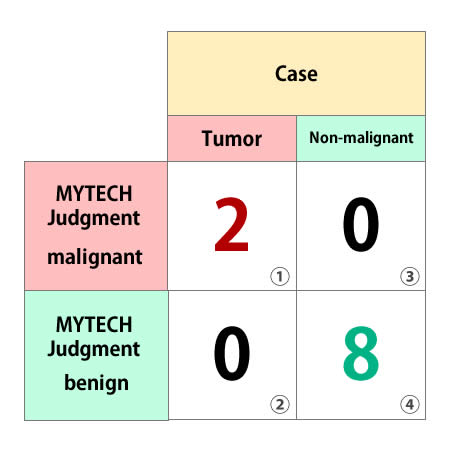
Sensitivity: 100.0%
* Probability that a sick person is correctly judged to be sick
calculation formula: ①÷(①+②)=1
calculation formula: ①÷(①+②)=1
Specificity: 100.0%
* Probability that a person who is not sick is correctly judged not to be sick
calculation formula: ④÷(③+④)=1
calculation formula: ④÷(③+④)=1
Accuracy rate 100.0%
* Overall correct answer rate without considering the type of incorrect answer
calculation formula: (①+④)÷①+②+③+④)=1
calculation formula: (①+④)÷①+②+③+④)=1
Patents owned by MYTECH
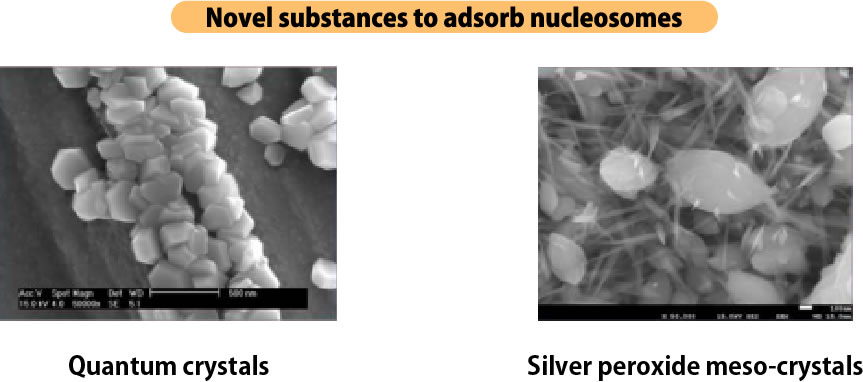
Novel substances developed by MYTECH as the first in the world.
They are increasing being ranted patents around the world, including Japan.
They are increasing being ranted patents around the world, including Japan.
Substance Patent
Quantum Crystal
A novel plasmonic substance with a new concept that Mitec has successfully developed for the first time in the world.
Metal complex crystals that self-assemble quantum dots in three dimensions.
Metal complex crystals that self-assemble quantum dots in three dimensions.
Silver peroxide meso-crystal
A novel plasmonic substance with a new concept that Mitec has successfully developed for the first time in the world.
Negatively charged nanocrystalline thin film of silver peroxide with oxygen content of 95% (atomic %), positively charged nucleosomes specifically bind to it.
Negatively charged nanocrystalline thin film of silver peroxide with oxygen content of 95% (atomic %), positively charged nucleosomes specifically bind to it.
Biochip Patent
Novel biochip that can detect cancer and disease in minutes.
Measurement Method Patent
Testing method for quantitatively measuring cancer-related substances Liquid biopsy measurement method using specific wavelengths for malignant and benign tumors.
Reference Literature, etc.
In 2014, Mytec published a paper on "Nanomedicine." (*1) as a method for detecting "cancer-derived substances (nucleosomes)" from blood, and in 2015, published a paper in "Scientific Reports" (*2).
In recent years, interest in the importance of nucleosomes has increased, and papers have been published by other research institutes. In 2016 "Cell." (*3), a research team at the University of Washington presented a method for inferring the pathological state of cancer by exffffamining nucleosomes.
Also, in 2019, a research team from RIKEN published in "Cell." (*4), and a research team from Johns Hopkins University published in "Nature." (*5) about the importance of nucleosomes.
In recent years, interest in the importance of nucleosomes has increased, and papers have been published by other research institutes. In 2016 "Cell." (*3), a research team at the University of Washington presented a method for inferring the pathological state of cancer by exffffamining nucleosomes.
Also, in 2019, a research team from RIKEN published in "Cell." (*4), and a research team from Johns Hopkins University published in "Nature." (*5) about the importance of nucleosomes.
*1 April 10, 2014 Nanomedicine. 2014 Apr;10(3):599-608.
「Use of surface-enhanced Raman scattering for detection of cancer-related serum-constituents in gastrointestinal cancer patients」
*2 May 21, 2015 Scientific Reports volume5, Article number: 10455 (2015)
「Silver Nanoscale Hexagonal Column Chips for Detecting Cell-free DNA and Circulating Nucleosomes in Cancer Patients」
*3 Jan. 14, 2016 Cell. 2016 Jan 14;164(1-2):57-68.
「Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin」
*4 Jan. 24, 2019 Cell. 2019 Jan 24;176(3):520-534.e25.
「Sub-nucleosomal Genome Structure Reveals Distinct Nucleosome Folding Motifs」
*5 May 29, 2019 Nature. 2019 May 29
「Genome-wide cell-free DNA fragmentation in patients with cancer」
*6 Research funded by a local independent administrative agency, the Osaka International Cancer Center Research project title (English):Hypersensitive diagnosis of urothelial cancer using silver nanoscale hexagonal column Period of study: 2016-2017 Principal Investigator: Kazuo Nishimura, Osaka International Cancer Center, Osaka Prefectural Hospital Organization of local independent administrative agency
*1 and *2: Papers published by MYTECH
* 3 to * 6: Papers published by other research institutes
「Use of surface-enhanced Raman scattering for detection of cancer-related serum-constituents in gastrointestinal cancer patients」
*2 May 21, 2015 Scientific Reports volume5, Article number: 10455 (2015)
「Silver Nanoscale Hexagonal Column Chips for Detecting Cell-free DNA and Circulating Nucleosomes in Cancer Patients」
*3 Jan. 14, 2016 Cell. 2016 Jan 14;164(1-2):57-68.
「Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin」
*4 Jan. 24, 2019 Cell. 2019 Jan 24;176(3):520-534.e25.
「Sub-nucleosomal Genome Structure Reveals Distinct Nucleosome Folding Motifs」
*5 May 29, 2019 Nature. 2019 May 29
「Genome-wide cell-free DNA fragmentation in patients with cancer」
*6 Research funded by a local independent administrative agency, the Osaka International Cancer Center Research project title (English):Hypersensitive diagnosis of urothelial cancer using silver nanoscale hexagonal column Period of study: 2016-2017 Principal Investigator: Kazuo Nishimura, Osaka International Cancer Center, Osaka Prefectural Hospital Organization of local independent administrative agency
*1 and *2: Papers published by MYTECH
* 3 to * 6: Papers published by other research institutes

